DIHYDROGEN, `H_2`
`color{green}(⧫)` `color{green}("Occurrence :")`
`color{green}(★)` Dihydrogen is the most abundant element in the universe (70% of the total mass of the universe) and is the principal element in the solar atmosphere.
`color{green}(★)` The giant planets Jupiter and Saturn consist mostly of hydrogen.
`color{green}(★)` However, due to its light nature, it is much less abundant (0.15% by mass) in the earth’s atmosphere.
`color{green}(★)` In the combined form it constitutes 15.4% of the earth's crust and the oceans.
`color{green}(★)` In the combined form besides in water, it occurs in plant and animal tissues, carbohydrates, proteins, hydrides including hydrocarbons and many other compounds.
`color{green}(⧫)` `color{green}("Isotopes of Hydrogen :")`
`color{green}(★)` Hydrogen has three isotopes: protium, `color{red}(text()_(1)^( 1)H)`, deuterium, `color{red}(text()_(1)^(2) H)` or `color{red}(D)` and tritium, `color{red}(text()_(1)^(3) H)` or `color{red}(T)`.
`color{green}(★)` These isotopes differ from one another in respect of the presence of neutrons.
`color{green}(★)` Ordinary hydrogen, protium, has no neutrons, deuterium (also known as heavy hydrogen) has one and tritium has two neutrons in the nucleus.
`color{purple}♣ color{Violet} " Just for Curious"`
In the year 1934, an American scientist, Harold C. Urey, got Nobel Prize for separating hydrogen isotope of mass number 2 by physical methods.
`color{green}(⧫)` The predominant form is protium.
`color{green}(⧫)` Terrestrial hydrogen contains `color{red}(0.0156%)` of deuterium mostly in the form of `color{red}(HD)`.
`color{green}(⧫)` The tritium concentration is about one atom per `color{red}(10^(18))` atoms of protium.
`color{green}(⧫)` Of these isotopes, only tritium is radioactive and emits low energy `color{red}(β^–)` particles (`color{red}(t_(1/2), 12.33)` years).
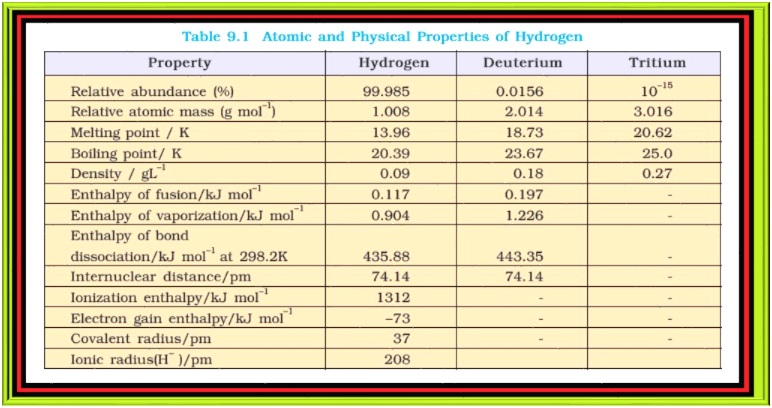
`color{green}(★)` Since the isotopes have the same electronic configuration, they have almost the same chemical properties. The only difference is in their rates of reactions, mainly due to their different enthalpy of bond dissociation (Table 9.1). However, in physical properties these isotopes differ considerably due to their large mass differences.
`color{green}(★)` Dihydrogen is the most abundant element in the universe (70% of the total mass of the universe) and is the principal element in the solar atmosphere.
`color{green}(★)` The giant planets Jupiter and Saturn consist mostly of hydrogen.
`color{green}(★)` However, due to its light nature, it is much less abundant (0.15% by mass) in the earth’s atmosphere.
`color{green}(★)` In the combined form it constitutes 15.4% of the earth's crust and the oceans.
`color{green}(★)` In the combined form besides in water, it occurs in plant and animal tissues, carbohydrates, proteins, hydrides including hydrocarbons and many other compounds.
`color{green}(⧫)` `color{green}("Isotopes of Hydrogen :")`
`color{green}(★)` Hydrogen has three isotopes: protium, `color{red}(text()_(1)^( 1)H)`, deuterium, `color{red}(text()_(1)^(2) H)` or `color{red}(D)` and tritium, `color{red}(text()_(1)^(3) H)` or `color{red}(T)`.
`color{green}(★)` These isotopes differ from one another in respect of the presence of neutrons.
`color{green}(★)` Ordinary hydrogen, protium, has no neutrons, deuterium (also known as heavy hydrogen) has one and tritium has two neutrons in the nucleus.
`color{purple}♣ color{Violet} " Just for Curious"`
In the year 1934, an American scientist, Harold C. Urey, got Nobel Prize for separating hydrogen isotope of mass number 2 by physical methods.
`color{green}(⧫)` The predominant form is protium.
`color{green}(⧫)` Terrestrial hydrogen contains `color{red}(0.0156%)` of deuterium mostly in the form of `color{red}(HD)`.
`color{green}(⧫)` The tritium concentration is about one atom per `color{red}(10^(18))` atoms of protium.
`color{green}(⧫)` Of these isotopes, only tritium is radioactive and emits low energy `color{red}(β^–)` particles (`color{red}(t_(1/2), 12.33)` years).
`color{green}(★)` Since the isotopes have the same electronic configuration, they have almost the same chemical properties. The only difference is in their rates of reactions, mainly due to their different enthalpy of bond dissociation (Table 9.1). However, in physical properties these isotopes differ considerably due to their large mass differences.

